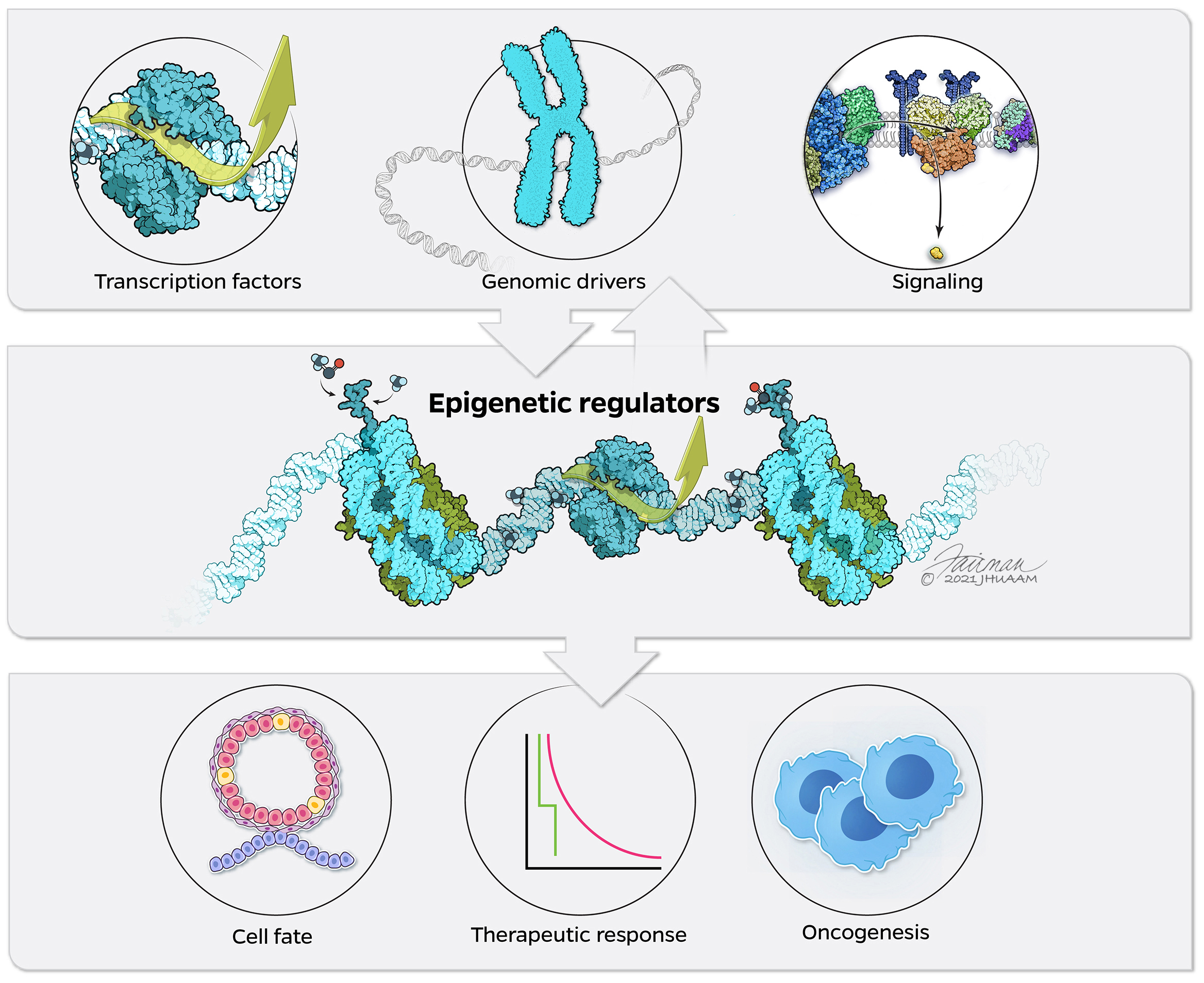
The crosstalk between signaling pathways and transcriptional regulation in hormone receptors driven cancers
Estrogen Receptor (ER) and the phosphoinositide 3-kinase (PI3K) pathways participate in regulatory crosstalk in breast cancer. The PI3K inhibitor alpelisib together in combination with ER therapy is now standard of care for the treatment of PIK3CA mutant/ER+ breast cancer. We identified that chromatin regulation is at the intersection of oncogenic PI3K and ER. The PI3K effectors AKT, and SGK (serum/glucocorticoid-regulated kinase) play a redundant role by phosphorylating the chromatin regulator KMT2D and modulating ER activity and therapy resistance (Toska et al, Science 2017; Toska et al Cell Reports 2019). Our studies underscore the relevance of the chromatin state in connecting the oncogenic signaling responses with gene activity. Our goal is to achieve a thorough and detailed understanding of the mechanisms of the cross-talks between epigenetic regulators and signaling pathways in hormone-driven cancers that can inform treatment strategy.
Role of epigenetic regulators in cancer progression, cell differentiation, and therapeutic response in cancer
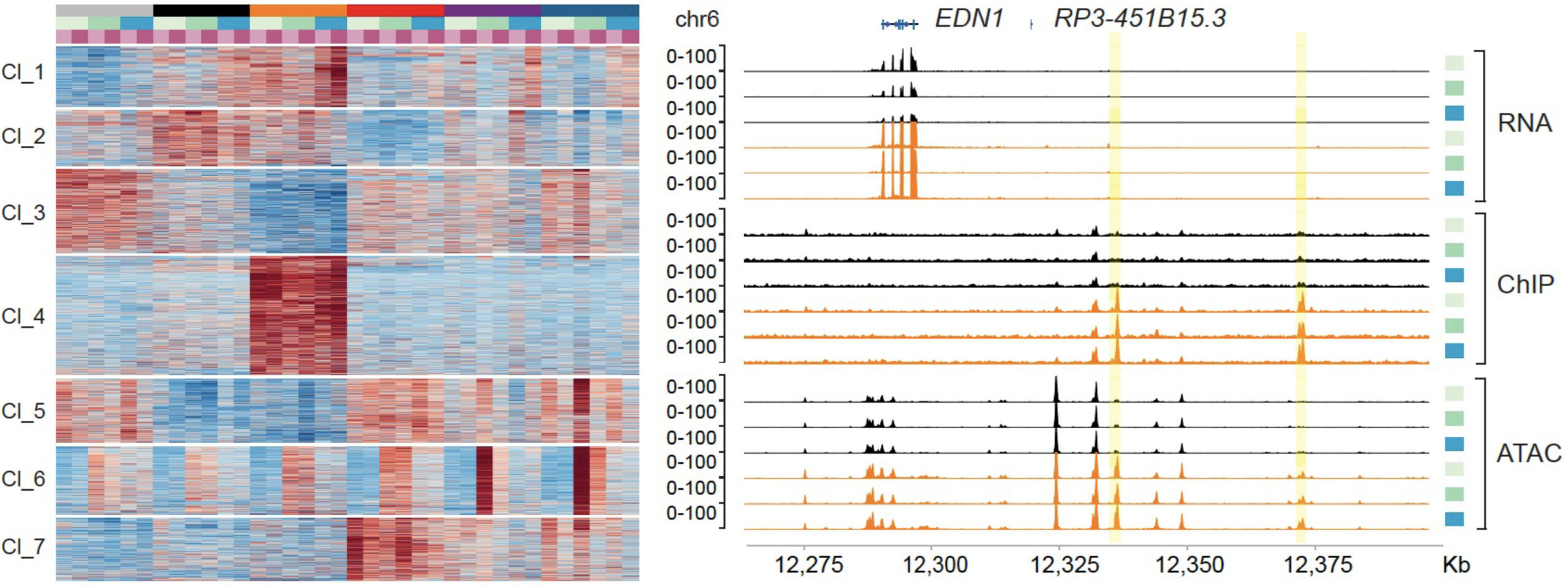
Approximately 70% of breast cancers express estrogen receptor (ER) and are treated with endocrine therapy. Emergence of resistance to these agents eventually develops in all patients with metastatic disease. Mechanisms of resistance, apart from recurrent mutations of ESR1 (gene coding for ER) are largely unknown. We have recently observed an enrichment of inactivating mutations in the genes encoding subunits of the SWI/SNF complex such as in ARID1A in endocrine-resistant metastatic breast cancer. Using an epigenome-wide CRISPR knockout screen and breast cancer patients samples harboring these mutations, we recently determined that ARID1A mediates endocrine resistance (Xu et al, Nature Genetics 2020). Moreover, we also found that mutations in the pioneer transcription factor FOXA1 are associated with worse outcome to endocrine therapy by inducing alternative chromatin profiles and gene activity in breast cancer (Arruabarrena-Aristorena et al, Cancer Cell 2020). We aim to understand the mechanisms by which these transcriptional regulators affect breast cancer progression and therapeutic response. Our ultimate goal is to determine the epigenetic drivers in tumorigenesis, and how mutations in epigenetic modifier genes and transcription factors alter transcription, increase lineage plasticity and tumor growth.